- Is Tea A Suspension Or Solution
- Suspension Or Solution Air
- Saltwater Suspension Or Solution
- Is Lemonade A Suspension Or Solution
- Suspension Or Solution
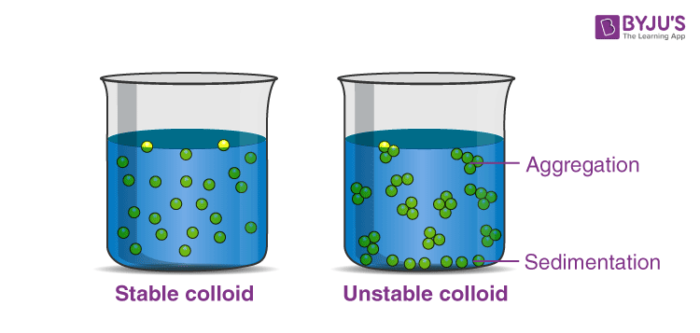
- : type of mixture that always looks cloudy because clumps of insoluble particles remain suspended throughout it – they don’t settle as sediment.
- A suspension is a heterogeneous mixture in which the solute particles do not dissolve, but get suspended throughout the bulk of the solvent, left floating around freely in the medium. The internal phase (solid) is dispersed throughout the external phase (fluid) through mechanical agitation, with the use of certain excipients or suspending agents.
What is mixtures, solutions and suspensions?
Finally, the solution will form two distinct layers where the top layer is less concentrated than the bottom one. For young children, most of the suspension used is liquid antibiotics. That is why patient education, in this case, is crucial.

Is Tea A Suspension Or Solution
Mixtures
A mixture is a combination of substances which are not chemically joined together.
- Mixtures have the same properties as their components
- There is no fixed proportion between the components
- The components can be separated from the mixture

Examples
Suspension Or Solution Air
- sugar and salt
- air with nitrogen and oxygen
Solutions
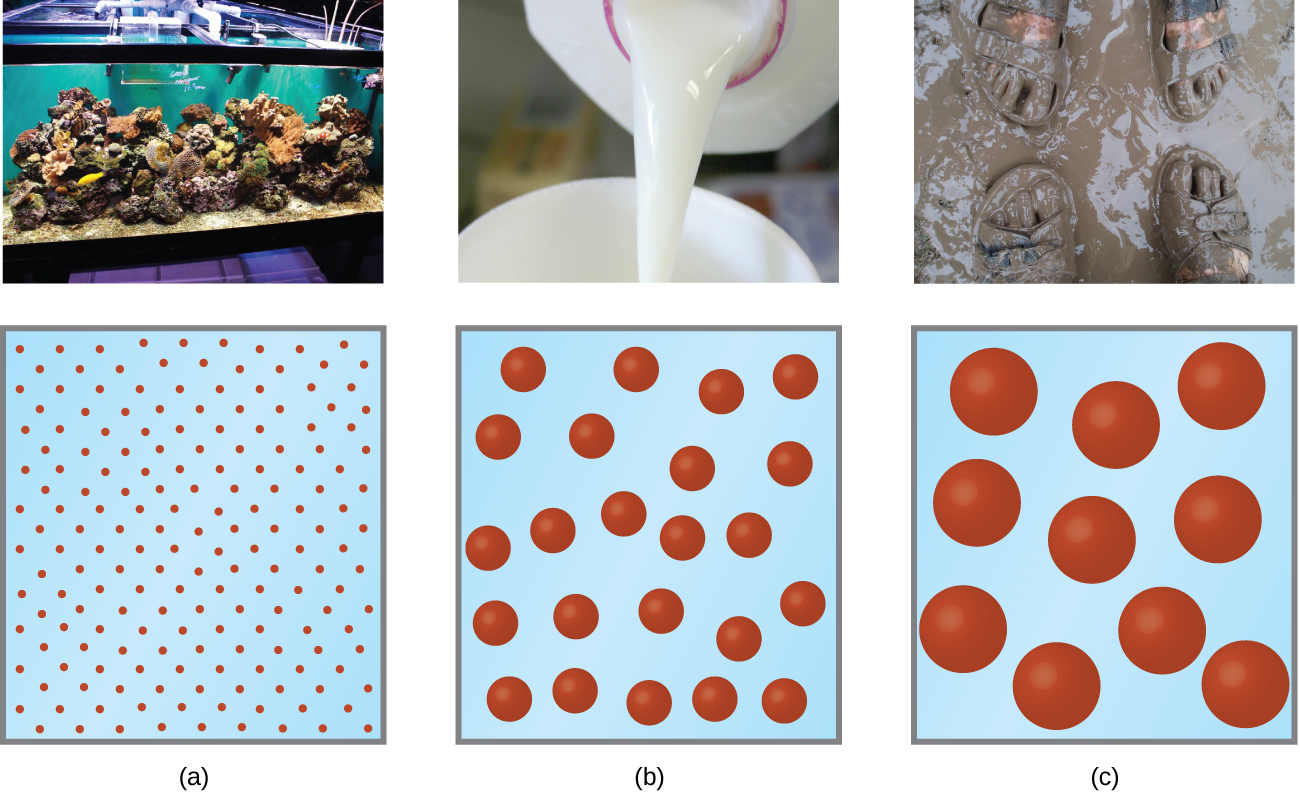
A solution is a homogeneous mixture in which one substance (solute) is dissolved in another substance (solvent).
- The components in a solution may not be separated from the solution by leaving it to stand, or by filtration
If a material dissolves in a liquid the material is said to be soluble. A solution is saturated if no more solute can be dissolved with temperature remaining constant.
Examples
- salt in sea water
Suspensions
A suspension is a mixture of liquids with particles of a solid which may not dissolve in the liquid.
- The solid may be separated from the liquid by leaving it to stand, or by filtration
Examples
Saltwater Suspension Or Solution
- sand in water
Related Topics
- Basics - The SI-system, unit converters, physical constants, drawing scales and more
- Fluid Mechanics - The study of fluids - liquids and gases. Involves velocity, pressure, density and temperature as functions of space and time

Related Documents
Is Lemonade A Suspension Or Solution
- Concentration Units Converter - Calculator and formulas for conversion between different units of concentration: Molarity, molality, mole fraction, weight percent of solute and grams of solute per liter of solution - descriptive terms for solubility
- Mixing Fluids - Final mass and temperature when mixing fluids
- Naming of inorganic binary compounds - Rules for naming inorganic ionic and covalent types of chemical compounds
- Shrinkage and estimation of density of a liquid-liquid solution - It is possible to estimate the density of a liquid-liquid solution from the density of the solute and the solvent. However, due to shrinkage, the estimate will be a bit too low.
- Solubility - A measure of the maximum amount of a solute that can be dissolved in a solvent
- Solubility guidelines for ionic compounds in water - Guidelines or solubility rules to predict whether or not a given ionic compound is soluble in water at room temperature
- Solubility product constants - The equilibrium constant, Ksp, for aqueous solutions of ionic compounds at 25°C.
- Solutions, molarity and dilution - Definitions and examples of how to calculate wt%, molarity and prepare dilutions
- Sugar Solutions - Viscosities - Dynamic viscosities of sucrose solutions at different temperatures
Suspension Or Solution
Tag Search
- en: mixtures solutions suspensions solubility