Introduction
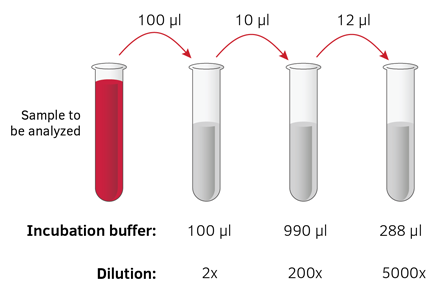
A Serial dilution is a series of dilutions, with the dilution factor staying the same for each step. The concentration factor is the initial volume divided by the final solution volume. The dilution factor is the inverse of the concentration factor. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; this has a concentration of 1/10th (0.1) of the original and a dilution factor of 10. These dilutions are often used to determine the approximate concentration of an enzyme (or molecule) to be quantified in an assay. Serial dilutions allow for small aliquots to be diluted instead of wasting large quantities of materials, are cost-effective, and are easy to prepare.

Equation 1.
[concentration factor= frac{volume_{initial}}{volume_{final}}nonumber]

[dilution factor= frac{1}{concentration factor}nonumber]
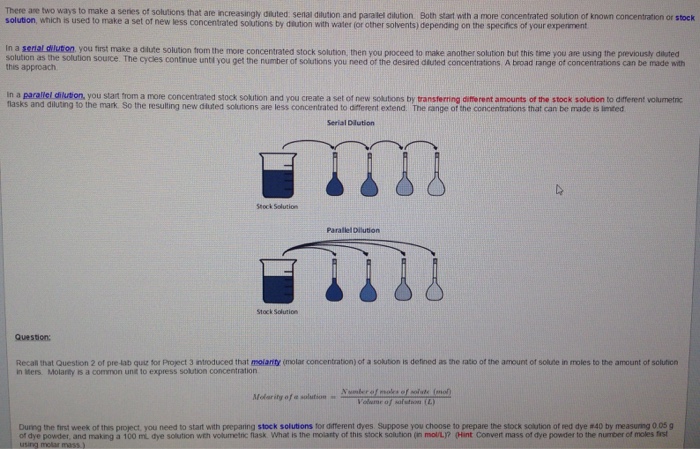
A serial dilution is the dilution of a sample, in 10-fold dilutions. As shown in the illustration below, it begins when 1 mL of the bacterial sample is added to 9 mL, and it is mixed together (creating a 10-1 dilution). Then, 1 mL from that mixture is added to 9 mL, and it is mixed together (a 10-2 dilution). Parallel dilution is the dilution of a solution with equal quantity of the same solvent with which the solution is made. E.g., 1mL of 100µg/ml strength aqueous solution can be diluted to 2mL of. Serial and Parallel Dilution A serial dilution is a stepwise preparation in which each dilution serves as the source for the next dilution.
Key considerations when making solutions:

The dilutions cover the range from 1/2 to 1/100 unevenly. In fact, the 1/2 vs. 1/3 dilutions differ by only 1.5-fold in concentration, while the 1/10 vs. 1/100 dilutions differ by ten-fold. If you are going to measure results for four dilutions, it is a waste of time and materials to make two of them almost the same.
- Make sure to always research the precautions to use when working with specific chemicals.
- Be sure you are using the right form of the chemical for the calculations. Some chemicals come as hydrates, meaning that those compounds contain chemically bound water. Others come as “anhydrous” which means that there is no bound water. Be sure to pay attention to which one you are using. For example, anhydrous CaCl2has a MW of 111.0 g, while the dehydrate form, CaCl2 ● 2 H2O has a MW of 147.0 grams (110.0 g + the weight of two waters, 18.0 grams each).
- Always use a graduate cylinder to measure out the amount of water for a solution, use the smallest size of graduated cylinder that will accommodate the entire solution. For example, if you need to make 50 mL of a solution, it is preferable to use a 50 mL graduate cylinder, but a 100 mL cylinder can be used if necessary.
- If using a magnetic stir bar, be sure that it is clean. Do not handle the magnetic stir bar with your bare hands. You may want to wash the stir bar with dishwashing detergent, followed by a complete rinse in deionized water to ensure that the stir bar is clean.
- For a 500 mL solution, start by dissolving the solids in about 400 mL deionized water (usually about 75% of the final volume) in a beaker that has a magnetic stir bar. Then transfer the solution to a 500 mL graduated cylinder and bring the volume to 500 mL
- The term “bring to volume” (btv) or “quantity sufficient” (qs) means adding water to a solution you are preparing until it reaches the desired total volume
- If you need to pH the solution, do so BEFORE you bring up the volume to the final volume. If the pH of the solution is lower than the desired pH, then a strong base (often NaOH) is added to raise the pH. If the pH is above the desired pH, then a strong acid (often HCl) is added to lower the pH. If your pH is very far from the desired pH, use higher molarity acids or base. Conversely, if you are close to the desired pH, use low molarity acids or bases (like 0.5M HCl). A demonstration will be shown in class for how to use and calibrate the pH meter.
- Label the bottle with the solution with the following information:
- Your initials
- The name of the solution (include concentrations)
- The date of preparation
- Storage temperature (if you know)
- Label hazards (if there are any)
Lab Math: Making Percent Solutions
Equation 2.
Formula for weight percent (w/v):
[ dfrac{text{Mass of solute (g)}}{text{Volume of solution (mL)}} times 100 nonumber ]
Serial Vs Parallel Dilution Method Definition
Example
Make 500 mL of a 5% (w/v) sucrose solution, given dry sucrose.
Serial Vs Parallel Dilution Method Formula
- Write a fraction for the concentration [5:%: ( frac{w}{v} ): =: dfrac{5: g: sucrose}{100: mL: solution} nonumber]
- Set up a proportion [dfrac{5: g: sucrose}{100: mL: solution} :=: dfrac{?: g: sucrose}{500: mL: solution} nonumber]
- Solve for g sucrose [dfrac{5: g: sucrose}{100: mL: solution} : times : 500 : mL : solution : = : 25 : g : sucrose nonumber]
- Add 25-g dry NaCl into a 500 ml graduated cylinder with enough DI water to dissolve the NaCl, then transfer to a graduated cylinder and fill up to 500 mL total solution.